The practice of Hadi Zambarakji
The practice of Hadi Zambarakji

Recent trends in the management of maculopathy secondary to pathological myopia
Pathological myopia – Prevalence and pathogenesis
Pathological or degenerative myopia, usually defined as a spherical equivalent refractive error of ≥-6 dioptres (D) or an axial length of ≥25.5mm, is one of the leading causes of visual loss in the world. The prevalence of pathological myopia (PM) is known to differ between ethnicities. In the adult Asian population its prevalence is 9-21%, compared to 2.8-4.6% in the U.S and Australia and the prevalence is thought to be rising globally. Myopic maculopathy describes a spectrum of clinical changes that comprise the main cause of visual loss among highly myopic individuals.
Myopic maculopathy
Long term studies suggest that approximately 40% of highly myopic eyes develop progressive maculopathy and over ten years more than half of these individuals will lose two lines of Snellen acuity. In addition to progressive maculopathy, macular hole, foveo-schisis and epiretinal membrane formation are much more common and often represent surgically challenging cases. (Figure 1)
Myopic macular choroidal neovascularisation (CNV) occurs in 4% to 11% of patients with high myopia and is the most common vision threatening complication of high myopia. It is the leading aetiology of CNV among patients younger than 50 years of age. In its advanced stage, CNV appears as a Fuchs’ spot which is a macular scar with pigment clumping and hyperpigmentation in association with fibrosis and retinal atrophy. Among myopic patients with pre-existing CNV, more than 30% will develop CNV in the fellow eye within 8 years.
Although natural history studies of untreated myopic CNV have presented conflicting evidence on visual prognosis, the overall trend suggests that long term visual prognosis with observation alone is poor. Patients over 50 years with myopic CNV observed for a mean of 4 years had a final visual acuity (VA) of 20/200 or worse in over two thirds of cases, and a 10 year follow up observational study noted that 96% of eyes achieve a final VA of 20/200 or worse. Thus active interventions are usually recommended for patients with myopic CNV.
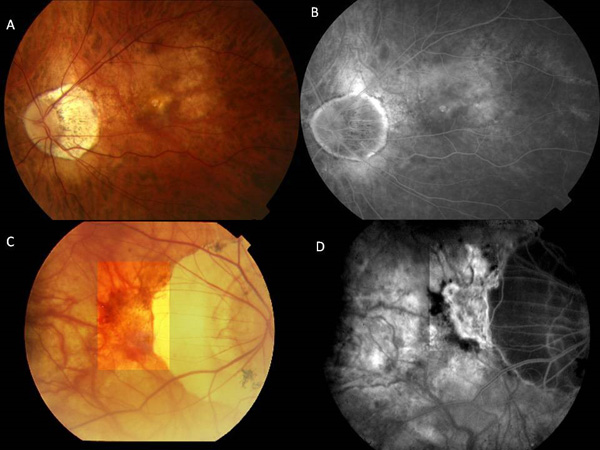
Figure 1 – Fundus images of 2 patients demonstrating the tessellated appearance (A), attenuated vasculature and peripapillary atrophy with associated retinal hemorrhage (C). The corresponding fluorescein angiograms demonstrate non specific hyperfluorescence in areas of mild atrophy of the retinal pigment epithelium and choriocapillaris (B) and intense peripapillary hyperfluoresce indicating the presence of a choroidal neovasucular membrane adjacent to extensive juxtapapillary chorioretinal atrophy (D).
Management of CNV secondary to pathological myopia
Myopic CNV has been treated with different approaches including laser photocoagulation, radiotherapy, submacular surgery, photodynamic therapy (PDT), ICG-mediated photothrombosis, macular translocation, combined PDT and intravitreal triamcinolone, transpupillary thermotherapy (TTT), and intravitreal injections of anti- vascular endothelial growth factor (VEGF) agents.
Direct laser photocoagulation is of limited benefit in myopic CNV.
Photodynamic therapy (PDT) with verteporfin causes selective damage to the choriocapillaris, sparing the neurosensory retina and retinal pigment epithelium (RPE) and has been used to treat subfoveal CNV without the complications of laser photocoagulation. Although some improvements were shown with combination intraocular triamcinolone and PDT in patients with subfoveal myopic CNV, there were significant adverse effects associated with intra-ocular steroid (triamcinolone) use and subsequent studies have demonstrated the added effect of combination therapy to be insignificant. PDT is considered to be of limited benefit in myopic CNV.
To date one study has compared intra vitreal Avastin versus Lucentis for the treatment of myopic CNV however it found no statistical difference between both treatment arms. A phase II multicentre open label trial of Ranibizumab for the Treatment of Choroidal Neovascularisation Secondary to Pathological Myopia: an Individualized Regimen (REPAIR) has reported its data in 2015 and provided definitive evidence of good patient satisfaction and well-being during treatment with Ranibizumab in myopic CNV.
Anti-VEGF Therapy: The role of VEGF as a regulator of angiogenesis has been the subject of much investigation. It is clear that ocular angiogenesis is a complex cascade that involves numerous proteins and biochemical interactions with VEGF playing a central role and its inhibition being an important therapeutic strategy. Anti-VEGF therapy with Avastin or Lucentis is considered the mainstay of treatment for myopic CNV.
Bevacizumab (Avastin) is an anti-VEGF antibody that neutralises all human forms of VEGF. Bevacizumab was developed for intravenous administration and approved for the treatment of cancer.
Bevacizumab is administered by intravitreal (intraocular) injection in several eye conditions. In 2006 reports began emerging of the beneficial use of Avastin in myopic CNV. By 2009, it was considered that, anti-VEGF treatment with Ranibizumab (Lucentis) or Bevacizumab (Avastin) can be considered as the first line recommended therapy for the treatment of CNV secondary to PM.
Overall, Avastin studies show that between 40 – 72% of cases demonstrate an improvement of more than 3 lines of VA at final follow up. No significant adverse effects were noted and the improvement in VA was seen to persist for up to two years. However the recommended number and time interval for treatments remains inconclusive, with some studies having administered only 1 injection, and others using an initial therapeutic treatment of 3 consecutive monthly injections. Younger patients appear to achieve a better final visual outcome and require fewer injections. Several authors have directly compared PDT and intra-vitreal Avastin, and all report the superior efficacy of Avastin.
Ranibuzumab (Lucentis) is an anti-VEGF antibody fragment that also neutralises all forms of VEGF. It is currently licensed by the U.S. Food and Drug Authority and the Committee for Medicinal Products for Human Use of the European Medicines Agency for the treatment of CNV secondary to age related macular degeneration. It has been shown to be effective in CNV secondary to PM, ocular histoplasmosis and angioid streaks.
There are several short term reports of effective Lucentis use in myopic CNV. Konstantinidis et al report that after an 8 month follow up, 64% (9/14 eyes) gained 3 or more lines of vision. Several one year follow-up reports have shown a similar trend. One study reported one year results whereby after a mean of 1.5 injections in 23 eyes, 69% of patients had improved vision of at least one line and 34.7% achieved three or more lines. Similarly, Silva et al report that out of 34 eyes with myopic CNV treated with a mean of 3.6 injections, at one year, 24% of the eyes improved ≥ 3 lines of VA, 44% improved ≥ 2 lines and 65% improved ≥ 1 line.
To date one study has compared intra vitreal Avastin versus Lucentis for the treatment of myopic CNV however it found no statistical difference between both treatment arms. Presently, a phase II multicentre open label trial of Ranibizumab for the Treatment of Choroidal Neovascularisation Secondary to Pathological Myopia: an Individualized Regimen (REPAIR) (www.clinicaltrials.gov – NCT01037348) is recruiting and may provide definitive evidence and a suitable regimen for the use of Ranibizumab in myopic CNV.
Surgical approaches to macular disease secondary to pathological myopia
High myopia accompanied by degenerative changes in the posterior segment and visual dysfunction may lend itself to surgical correction in some patients, however severe axial elongation of the globe, the presence of a posterior staphyloma and atrophy of the RPE and choroid make these cases challenging. Surgical approaches to the management of myopic macular CNV have involved primarily surgical excision of CNV and macular translocation. Other causes of visual dysfunction in PM that have been managed surgically include include myopic macular hole (MMH), retinal detachment associated with macular hole, posterior staphyloma and macular foveoschisis with preretinal traction.
Surgical excision of CNV and macular translocation are generally considered older treatments and have been superseeded by intravitreal therapy with Lucentis and Avastin.
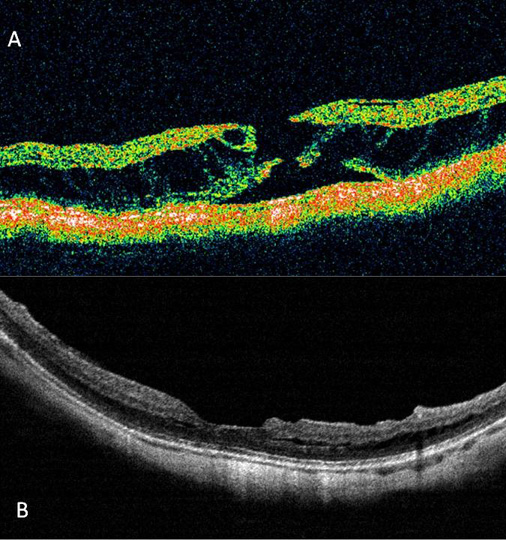
Macular foveoschisis (MF) (also termed retinoschisis) is thought to occur in 9-20% of highly myopic eyes with posterior staphyloma. The OCT features of MF comprise retinal thickening and splitting of the neurosensory retina into a thin outer outer layer and a thicker reflective inner layer, however an inner retinoshisis may also be present in some eyes. (Figure 2) This can be associated with a foveal cyst which can be partially deroofed giving the appearance of a lamellar hole and in some patients a foveal detachment is present. A natural history study of 9 eyes with MF without preretinal membranes over at least one year demonstrated no significant progression. However, a macular hole may develop spontaneously or possibly after vitreous surgery as shown in 9 of 29 eyes reported by Gaucher et al.
The surgical outcomes of vitreous surgery for MF and foveal detachment without macular hole have been shown to produce significant anatomical improvements and moderate improvements in vision. Kobayashi et al in a series of 9 eyes had post operative LogMAR visual acuities of 0.4 to 0.6, and similarly Scott et al reported post operative visual acuities of 20/40 to 20/80 in 3 eyes. A small risk of post operative macular hole formation has been reported and this is thought to be associated with the presence of premacular traction membranes.
Furthermore, the possible value of stopping axial elongation in PM using macular buckling surgery has been reported, but the true value of this procedure in the context of MF as a prophylactic measure to prevent progressive change to macular hole with an associated retinal detachment has not been fully investigated.
In conclusion, well designed prospective studies with clear case definition are lacking. Nonetheless, limited evidence support a role for vitreous surgery with separation of the posterior vitreous cortex in eyes with progressive visual loss and MF associated with preretinal tractional membranes. The removal of all tractional forces may underlie the reported benefit, however the true value in peeling the internal limiting membrane remains unclear. The prophylactic role of macular buckling deserves further investigation.
Figure 2 – (A) A pre-operative TD-OCT on a patient with foveoschisis and full thickness macular hole and (B) SD-OCT one year post vitrectomy and ILM peel, demonstrating closure of the macular hole but persistent schisis.
Macular hole (MH) and associated retinal detachment (RRD) are rare in the general population. They occur principally in highly myopic eyes and are thought to be the result of tangential vitreoretinal traction.
The reported closure rates for MH associated with RD and PM are disappointing. Pars plana vitrectomy (PPV) in combination with inner limiting membrane (ILM) peel and a long or short acting gas tamponade is commonly used to repair MH. Reported closure rates using the above approaches vary between 10% and 44% in studies that have used OCT to evaluate MH closure in high myopes. Reported closure rates also vary significantly if OCT is not used as part of the macular assessment. Whilst MH closure rates are poor, retinal reattachment rates following vitrectomy surgery are thought to be more successful, varying between 40-93%.
Most published data on the role of vitrectomy for myopic MH and retinal detachment have included peeling of preretinal structures and/or the ILM. To our knowledge, there are no randomised trials that have evaluated the role of epiretinal membrane or ILM peel and no studies that have compared types of intraocular tamponade. It is however intuitive to assume that complete peeling of the thickened posterior hyaloid and all pre retinal structures constitutes a logical approach given the postulated pathogenic forces involved.
Vitrectomy alone however does not address the presence of a posterior staphyloma which has been strongly associated with retinal detachment secondary to MH. In order to treat the posterior staphyloma, macular buckling (MB) was developed. MB is not a new surgical technique but it has not been widely adopted. Thus reports of surgical outcomes of MB for myopic MH associated RD are limited. Reported retianl reatachment rates var between 79-93% and MH closure in 10 of 12 eyes (83%) were reported by Ando et al where the macular buckle produced an indent over the MH. Based on the available evidence, most of which relates to short term case series, it would be difficult to select the optimal surgical technique for managing retinal detachment secondary to myopic MH. There is significant heterogeneity between different studies which highlights the need for a well designed randomised trial to evaluate the role of vitrectomy surgery and ILM peeling for myopic MH and secondary retinal detachment. Furthermore, the role of MB remains inconclusive and this surgical technique deserves further evaluation for the management of MH and associated retinal detachment in PM.
Conclusions
- The natural history and long-term visual prognosis of untreated CNV in myopic patients is very poor.
- The efficacy of laser, PDT and macular translocation has been superseded by the use of intra-vitreal anti-VEGF agents which are currently considered as first line therapy for myopic CNV. Nonetheless, large scale randomised controlled trials are necessary to identify the most suitable agent and to establish an appropriate frequency and dosing regimen. Certain recent developments such as VEGF trap (Eylea) have shown early promise in the treatment for myopic CNV however further evaluation of safety and benefit are needed.
- Macular foveoschisis associated with epimacular traction and visual decline as well as macular hole with secondary retinal detachment are both surgically treatable conditions. Although well designed studies are lacking, several interventional series highlight a clinical benefit in surgical intervention. Treatment for macular hole with associated retinal detachment should be done promptly.
- Macular buckling is a procedure that reduces the progression of axial elongation and the tractional effects of a posterior staphyloma however, a clear and repeatable benefit is yet to be demonstrated. Randomised studies are therefore necessary to develop a uniform evidence-based approach for treatment.
Important notes for the patient before intravitreal injections:
Risk when Avastin is given to treat patients with eye conditions
Ophthalmologists believe that the risk of serious complications for patients with eye conditions is very low. Patients receiving Avastin for eye conditions are healthier than those treated with Avastin for cancer, and receive a significantly smaller dose, delivered only to the cavity of the eye. While there are no approved studies for the use of Avastin in the eye that prove it is safe and effective, Lucentis, a similar drug, is approved for macular degeneration. One study of patients who received Avastin through an intravenous infusion reported only a mild elevation in blood pressure. Another study of patients treated with intravitreal> Avastin (that is, Avastin injected into the eye) did not have these elevations or the other serious problems seen in the patients with cancer.
Lucentis is approved for the use by the intravitreal route but Avastin is not approived for use by the intravitreal route.
However, the benefits and risks of intravitreal Avastin for eye conditions are not yet fully known. In addition, whenever a medication is used in a large number of patients, a small number of coincidental life-threatening problems may occur that have no relationship to the treatment. For example, patients with diabetes are already at increased risk for heart attacks and strokes. If one of these patients being treated with Avastin suffers a heart attack or stroke, it may be associated with the diabetes and not necessarily the Avastin treatment.
Known risks for intravitreal eye injections
Your condition may not get better or may become worse. Any or all of these complications may cause decreased vision and/or have a possibility of causing blindness. Additional procedures may be needed to treat these complications. During the follow up visits, you will be checked for possible side effects and the results will be discussed with you.
Possible complications and side effects of the procedure and administration of Avastin include but are not limited to retinal detachment, cataract formation (clouding of the lens of the eye), glaucoma (increased pressure in the eye), hypotony (reduced pressure in the eye), damage to the retina or cornea (structures of the eye), and bleeding. There is also the possibility of an eye infection (endophthalmitis). You may receive eye drops with instructions on when to use them to reduce the possibility of this occurring. Any of these rare complications may lead to severe and permanent loss of vision.
Patients receiving an intravitreal injection may experience less severe side effects related to the pre-injection preparation procedure. These side effects may include eye pain, subconjunctival hemorrhage (bloodshot eye), vitreous floaters, swelling of the cornea, inflammation of the eye, and visual disturbances.
PATIENT RESPONSIBILITIES
I will urgently contact my ophthalmologist if any of the following signs of infection or other complications develop: pain, blurry or decreased vision, sensitivity to light, redness of the eye (compared to immediately after the injection), or discharge from the eye. I have been instructed NOT to rub my eyes or swim for three days after each injection.
Although the likelihood of serious complications affecting other organs of my body is low, I will urgently contact my General Practitioner or go to the hospital casualty if I experience abdominal pain, chest pain, severe headache, slurred speech, or weakness on one side of the body.
Prepared by Mr. H.J. Zambarakji FRCOphth, D.M
Consultant Ophthalmic surgeon
Search the RetinaCare website
Visual guides
 Vitreomacular traction and small macular hole successfully treated with intravitreal Ocriplasmin
Vitreomacular traction and small macular hole successfully treated with intravitreal Ocriplasmin
 Macular hole (pre and post op)
Macular hole (pre and post op)
 Epiretinal membrane (pre and post op)
Epiretinal membrane (pre and post op)
 Diabetic retinopathy (medical management and laser photocoagulation)
Diabetic retinopathy (medical management and laser photocoagulation)
 Traction diabetic retinal detachment (pre and post op)
Traction diabetic retinal detachment (pre and post op)
READ HADI’S LATEST NEWS